Purpose Of Serial Dilution Method
Posted By admin On 23.01.20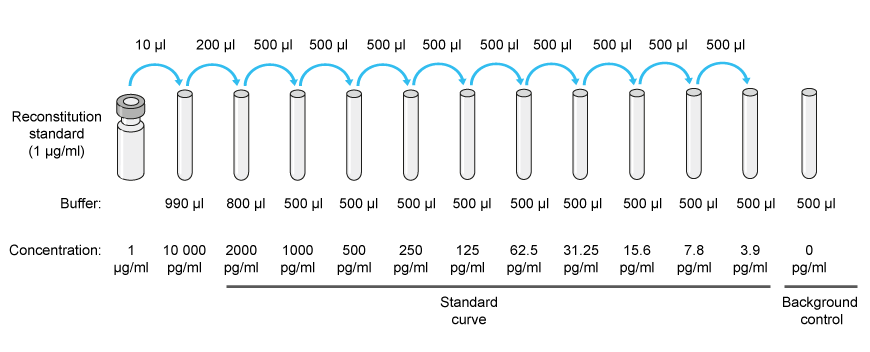
Serial dilution is usually 1/10 dilution. Therefore after aseries of dilutions, you have a logarithmic curve of concentration(log10). Basically, if diluting 1/10 and starting off with 1 molarsolution, first dilution = 0.1M, 2nd = 0.01M, 3rd = 0.001M. Ifmaking a 0.001M solution involved weighing out 0.005g of a salt forexample, the error in making this solution out would be very largein comparison to weighing out 5g (1M) and diluting it 3 times byserial dilution. The benefit of it is mainly accuracy.
Titration of microorganisms in infectious or environmental samples is a corner stone of quantitative microbiology. A simple method is presented to estimate the microbial counts obtained with the serial dilution technique for microorganisms that can grow on bacteriological media and develop into a colony.
Serial Dilution Experiment
The number (concentration) of viable microbial organisms is estimated from a single dilution plate (assay) without a need for replicate plates. Our method selects the best agar plate with which to estimate the microbial counts, and takes into account the colony size and plate area that both contribute to the likelihood of miscounting the number of colonies on a plate.
The estimate of the optimal count given by our method can be used to narrow the search for the best (optimal) dilution plate and saves time. The required inputs are the plate size, the microbial colony size, and the serial dilution factors. The proposed approach shows relative accuracy well within ± 0.1 log 10 from data produced by computer simulations.

The method maintains this accuracy even in the presence of dilution errors of up to 10% (for both the aliquot and diluent volumes), microbial counts between 10 4 and 10 12 colony-forming units, dilution ratios from 2 to 100, and plate size to colony size ratios between 6.25 to 200.